Oxcarbazepine ER Suspension: Development of In Vitro Dissolution Profile
An in-depth case study on the development of the in vitro dissolution profile for Oxcarbazepine ER suspension, enhancing drug formulation and efficacy.
Development of Oxcarbazepine ER Suspension Dissolution Profile
Objectives
To establish dissolution profile of Oxcarbazepine (OXC) ER dosage form establishing BE when given QD with Trileptal IR suspension given BID, at steady state
Background
- OXC is approved as monotherapy or adjunctive therapy for partial seizures in adults or in children ≥ 4 yr of age
- Currently marketed as Trileptal® with 150, 300, 600 mg tablets and 60 mg/ml (6%) oral suspension
- The Tmax of OXC is 1-3 h and its active metabolite’s (MHD) 4-12 h
- At SS peak levels of MHD occurs at 2-4 h
- Elimination t1/2 of OXC is 1-5 h and MHD is 7-20 h for MHD
- Metabolism of OXC to MHD occurs in liver and excreted majorly through renal route
- There is no auto-induction observed for OXC
Characterizing PK profile of IR suspension of 600mg OXC
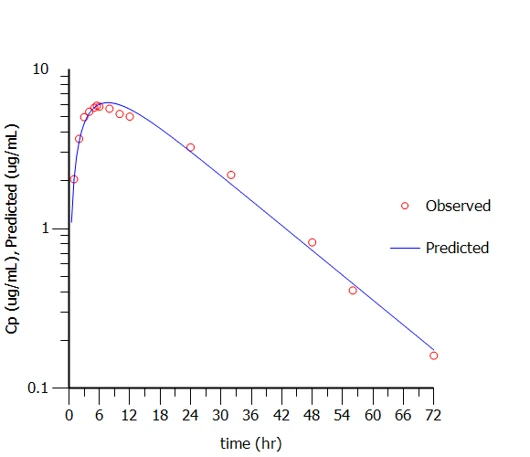
- Trileptal suspension’s mean PK profile (of MHD) was characterized
- An 1-compartment model with 1st-order elimination described the plasma profile
| PARAMETER | ESTIMATE | CV% |
|---|---|---|
| V/F(mL) | 585211 | 5.1 |
| KO1(1/h) | 0.423 | 10 |
| CL/F(Ml/h) | 3626 | 3.3 |
| K10 (1/h) | 0.062 | 2.8 |
- The model derived Cmax and AUC are within 5% of the actual profile’s PK values (from SBA)
- The modeled Tmax and t1/2 are ~ 20 min and 10 min different than the profile values
- The derived initial estimates will aid in establishing unit impulse response (UIR) and weighting functions for convolution
Steady state (SS) simulation and PK comparison vs. reported SS study
- The modeled profile is simulated to steady state with BID dosing given for 6 days
- There is a prediction error of 28-36% in all the PK parameters at SS
| PARAMETER | MODELED | ACTUAL | %PE |
|---|---|---|---|
| Cmax,ss(ng/mL) | 14.7 | 23.0 | 36 |
| AUCt(ng/mL) | 163 | 231 | 29 |
| Cmin,ss (ng/mL) | 11.6 | 16.1 | 28 |
| Tmax(h) | 7.6 | 4.0 |
- This could be related to saturation of metabolism of OXC to MHD in liver with repeated dosings (Theisohn M et al, 1982)
- There is an accumulation of 1.4 (=AUCƬ/AUCinf) from the actual steady state vs. single dose study

Building dissolution model of ER suspension
- To match the actual Cmin,ss (16 ug/mL), we calculated the change in K10 iteratively by using:
Cmin,ss = D/V*(1/(1-e^K10*Ƭ))*(e^K10)
- A K10=0.039 h-1 (~t1/2 = 17.7 h) was found to match the study-derived Cmin,ss
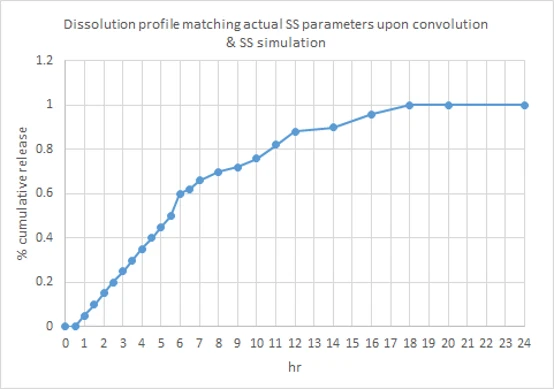
Dissolution profile of 1200 mg ER suspension was selected iteratively, which upon convolution (translating in vitro to in vivo profile) & SS simulation can match the PK parameters of actual SS study.
Convolution & SS simulation of dissolution profile and comparison vs IR suspension
- The SS simulation of 1200 mg QD derived convolution profile considered 3 scenarios:
- Taking all the initial estimates unchanged as obtained from PK model of 600mg IR suspension
- Changing only K10 from modeled parameter (0.06 h-1) to 0.039 h-1 (from derived Cmin,ss)
- Changing K10=0.039 h-1 and k01=0.34 (derived from the SS Tmax=4 h opposed to single dose Tmax=6 h)
- The simulation derived PK profile according to 3 scenarios were compared against actual SS study derived PK profile
- Both scenarios ii) and iii) matches the SS profiles of IR suspension
Assumption: Due to saturation of conversion from OXC to MHD, the clearance of OXC diminishes by repeated dosing resulting into longer t1/2’s and shorter Tmax and increased exposure;
Hence, with changing PK upon multi-dosing, the profile will undergo through all the 3 scenarios to achieve exposure meeting BE

In vitro data of Oxtellar XR
- From Oxtellar XR’s SBA – it was found that IVIVC model was developed but it could not be validated
- It could be due to selection of wrong discriminatory media or due to higher variabilities observed between the batches both in vitro and/or in vivo
- The multi-media dissolution read-outs were convoluted with the UIR obtained for MHD from Trileptal 600 mg tablets to obtain the in vivo profiles

Finding the right discriminatory media

- In vivo profile obtained for each disso. media were simulated for steady state (SS) profiles
- The PK parameters of simulated SS profiles were compared against Trileptal 300 mg BID at SS
- It was observed that the 0.1 N HCl media approximately characterizes the Oxtellar XR’s SS profile – discriminatory media ?
- Diffrence in Cmin and AUCtau between the simulated & actual Oxtellar could be due to non-linearity and accumulation* under real SS condition compared to linear PK assumption of our SS simulation
- We attempted validation of discriminatory media through confirmatory checks
Confirmatory check for discriminatory media
- The Oxtellar XR’s single dose OXC profile was simulated in its absence
- PK parameters of OXC was within ±15% of the reported means (and were well within the actual mean ± SD’s)
- The simulated conc.-time profile of OXC from Oxtellar XR was deconvoluted by OXC profile from Trileptal
- The deconvoluted profile looks comparable to the in vitro data at 0.1 N HCl media
- Therefore, 0.1 N HCl seems to be the discriminatory media for ER formulations



Predicting the disso.profile meeting BE to Trileptal BID
- With multiple iterations the ER dissolution profile was simulated
- It was estimated to be a profile in 0.1N HCl with 1% SLS, releasing drug at 20% in 2 h, 50% in 4 h, 80% in 8 h, 90% in 10 h and remaining at ~ 12 h
- The simulated ER disso. profile was convoluted to find in vivo profile
- The in vivo 600mg profile was compared for BE to Trileptal 300 mg BID
- The dose can be changed accordingly for ER formulation provided the same dose given in BID for Trileptal
Who We Are
Pioneering Drug Development: Biopharmetrics Consultancy
At Biopharmetrics, we are dedicated to empowering the pharmaceutical industry through our consultancy services. With a focus on navigating uncertainties in research and regulatory inquiries, we provide expert guidance that fosters confidence in the development of groundbreaking medicines, ultimately enhancing the lives of patients worldwide.



















Frequently Asked Question?
Understanding Our Services Through Case Studies
How can Biopharmetrics assist in the development of in vitro dissolution profiles for pharmaceutical formulations like Oxcarbazepine ER Suspension?
Biopharmetrics offers comprehensive support in developing in vitro dissolution profiles for pharmaceutical formulations such as Oxcarbazepine ER Suspension. Through meticulous analysis and simulation techniques, we assist in establishing dissolution profiles that enhance drug formulation efficacy. Our process involves iterative modeling to match actual pharmacokinetic (PK) parameters, ensuring accuracy and reliability in predicting drug behavior under various conditions.
What role does Biopharmetrics play in optimizing drug dosage forms to achieve bioequivalence (BE) with existing medications like Trileptal IR Suspension?
Biopharmetrics plays a pivotal role in optimizing drug dosage forms to attain bioequivalence with established medications like Trileptal IR Suspension. We employ sophisticated modeling techniques to simulate steady-state profiles, iteratively adjusting dissolution parameters to match desired pharmacokinetic outcomes. By ensuring bioequivalence, our solutions facilitate seamless transition and compatibility between different drug formulations, maintaining therapeutic efficacy and patient convenience.
How does Biopharmetrics support the selection of discriminatory media for in vitro dissolution testing, as demonstrated in the case of Oxtellar XR?
Biopharmetrics provides expertise in selecting discriminatory media for in vitro dissolution testing, as demonstrated in the case of Oxtellar XR. Through rigorous simulation and validation processes, we identify media that accurately mimic in vivo conditions, ensuring reliability in predicting drug behavior. Our approach involves comparative analysis of simulated profiles against actual pharmacokinetic parameters, enabling the precise selection of media that best represent drug performance, ultimately optimizing formulation development and regulatory compliance.
Testimonials
Discover What Our Clients Have to Say
We had an outstanding experience working with Biopharmetrics. Their deep expertise in PBPK Modelling & Simulation was evident from the start of the mAb project. The Clinical Pharmacology consultancy support in clarifying queries from the Regulatory Agency and Principal Investigator of our upcoming trial was helpful. The team’s dedication to our project, attention to detail and strict adherence to our timelines helped us achieve our goals ahead of schedule. They worked closely as part of the regular project team and took utmost care in proposing any solutions. We appreciate their commitment and highly recommend Biopharmetrics for anyone seeking PBPK Services." - Battery Bio at Vial

We engaged Biopharmetrics for IVIVC services for our products and continue to work with them, they have been very proactive in proposing solutions like optimizing the dissolution profile, selecting bio-relevant dissolution media based on pilot bio results and shortlisting the pilot batches for pivotal BE studies. Their experience in using mathematical modelling tools ie IVIVC and PBPK for predictions has helped us immensely. They have been an integral part of R&D projects, working closely with CFT’s and aligning the deliverables as per our timelines. We recommend Biopharmetrics to others who are experiencing challenges in their formulation R&D

Appcure Labs, USA& India

